Intermediate
+86-571-88278597
API
+86-571-88278599
FDF
+86-13336178889
.jpg)
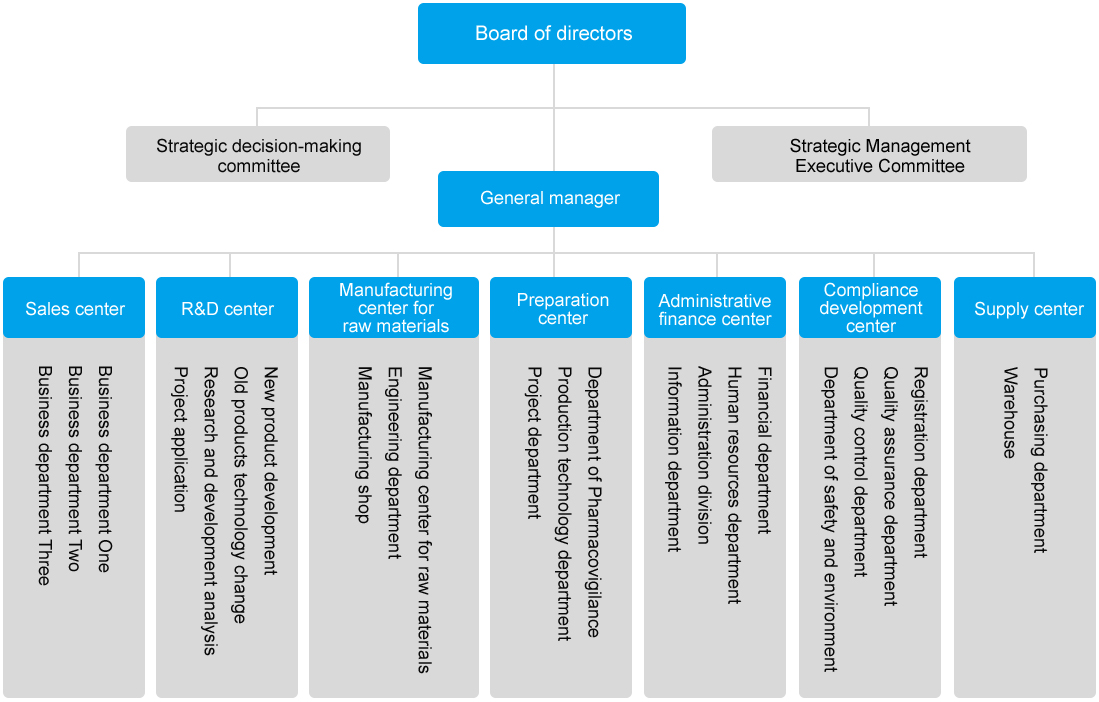
Zhejiang Hongyuan Pharmaceutical Co., Ltd. was founded in 2005. It is located in Linhai Taizhouwan Economic Development Zone, with about 430 employees. The company is a third-level member of "World Top 500" Property Zhongda Group Co., Ltd.
The company is committed to the development and production of preparations, apis and intermediates in the field of cardiovascular and cerebrovascular and hypoglycemia. The main products are Atorvastatin, Rosuvastatin calcium, Pitavastatin and Azisartan for the treatment of cardiovascular and cerebrovascular diseases, and Empagliflozin and Dapagliflozin for lowering blood sugar. The sales area covers Europe, India, the Middle East, Japan and South Korea and other countries and regions. The company has established standardized and perfect quality management and SHE management system, obtained the national GMP, EU CEP, Taiwan registration and other certificates, passed the safety standardization, clean production, ISO14001 environmental management system and ISO45001 occupational health and safety management system and TFS social responsibility certification. As a national high-tech enterprise, the company has been awarded the honors and qualifications of Zhejiang High-tech Enterprise Research and Development Center, Zhejiang Enterprise Technology Center, and Zhejiang Enterprise Research Institute, and has built Zhejiang Qinggong Innovation and Efficiency Studio, which is a special and special new enterprise in Zhejiang Province, an intellectual property demonstration enterprise in Zhejiang Province, and an advanced enterprise in Taizhou to create harmonious labor relations. The vice president unit of Linhai Hazardous Chemicals Association won the third prize of Zhejiang Science and Technology Progress Award in 2021. The company has established long-term project partnerships with well-known domestic universities and scientific research institutions such as Zhejiang University, Zhejiang University of Technology, Taizhou College, Taizhou Bio-medical Industry Research Institute, and hired a group of industry experts to serve as R & D guidance, adhere to technological progress and technological innovation, and constantly improve core competitiveness.
Adhering to the concept of "common development of enterprises and employees", the company is willing to work with you on the basis of mutual trust, mutual understanding and collaboration, and strive to build the company into an internationally renowned pharmaceutical enterprise with featured apis and preparations.